In
this study, we designed a novel drug-eluting coating for vascular
implants consisting of a core coating of the anti-proliferative drug
docetaxel (DTX) and a shell coating of the platelet glycoprotein
IIb/IIIa receptor monoclonal antibody SZ-21. The core/shell structure
was sprayed onto the surface of 316L stainless steel stents using a
coaxial electrospray process with the aim of creating a coating that
exhibited a differential release of the two drugs. The prepared stents
displayed a uniform coating consisting of nano/micro particles. In vitro
drug release experiments were performed, and we demonstrated that a
biphasic mathematical model was capable of capturing the data,
indicating that the release of the two drugs conformed to a
diffusion-controlled release system. We demonstrated that our coating
was capable of inhibiting the adhesion and activation of platelets, as
well as the proliferation and migration of smooth muscle cells (SMCs),
indicating its good biocompatibility and anti-proliferation qualities.
In an in vivo porcine coronary artery model, the SZ-21/DTX drug-loaded
hydrophobic core/hydrophilic shell particle coating stents were observed
to promote re-endothelialization and inhibit neointimal hyperplasia.
This core/shell particle-coated stent may serve as part of a new
strategy for the differential release of different functional drugs to
sequentially target thrombosis and in-stent restenosis during the
vascular repair process and ensure rapid re-endothelialization in the
field of cardiovascular disease.
Dichloromethane and ethylic acid solution were used as the solvents for PLGA and CS, respectively. In the electrospray coating process, 10 mL syringes containing DTX-PLGA solution (DTX concentration at 1 mg/mL) or SZ-21-CS solution (SZ-21 concentration at 2 mg/mL) were connected with a coaxial nozzle. The reception distance between the tip of the needle to the coating surface was maintained at 5–15 cm; the voltage and inner/outer flow rate were changed to obtain a uniform coating (Table S1). The SZ-21/DTX drug-loaded hydrophobic core/hydrophilic shell particle coating (DPC) and no drug core/shell particle coating (BPC) were prepared according to the following selected parameters: 0.2 mL/h (inner) and 0.6 mL/h (outer), a PLGA concentration of 10 mg/mL, a CS concentration of 2.5 mg/mL, a reception distance of 5 cm, and a voltage of 14 kV. The morphology, core/shell structure, and particle size distribution were detected through a fluorescence microscope (IX81, Olympus, Japan), scanning electron microscope (SEM; Vega-LMH, Tescan, Czech), confocal microscope (SP8, Leica, German) and transmission electron microscope (TEM, JEM-2100F, JEOL, Japan).
Each route of transport was associated with a different effective transport speed characterized by a rate constant ki, i = 1,2. Assuming that the drug was initially uniformly distributed within each respective route, the strut was impermeable to drug transport, the release medium acted as an infinite sink and the drugs were dilute, and then considering drug transport within each route as a diffusion-dominated process, we derived an expression for the mass of each drug within the coating as a function of time, M(t):
M(t)=∑n=1∞A(n){re−K1(n)t+(1−r)e−K2(n)t}
where A(n)=8M0π2(2n−1)2,K1(n)=(2n−1)2π2k14,K2(n)=(2n−1)2π2k24
, and r is the initial fraction of the total drug loading (M0) within each route.
Using the knowledge of the total drug loading for each drug and Eq. (1), we calculated the cumulative fraction of each drug that had been released as a function of time.
morphologicalindex=(No.typeI×1+No.typeII×2+No.typeIII×3+No.typeIV×4+No.typeV×5)totalnumberofadherentplatelets
The hemolysis ratio of DPC
was tested following the Chinese national standard (Biological
Evaluation of Medical Devices—Part 4: Selection of Tests for
Interactions with Blood, GB/T 16886.4-2003). The activated
partial thrombin time (APTT) and prothrombin time (PT) were applied to
evaluate the anti-thrombogenicity of stents in vitro37.
After a 5-day culture, the stents were immobilized with 2.5% glutaraldehyde and dehydrated for morphological observation by SEM. The proliferation rates of cells on the stent surfaces were investigated with Cell Titer 96® Aqueous One Solution Cell Proliferation Assay after 1, 3, and 5 days. The relative growth of rate (RGR) was calculated by this formula:
RGR=(ODtODnc)×100%
(where ODt represents the experimental group, and ODnc represents the negative control group).
After co-culturing for 1, 3, and 5 days, the culture supernatant was collected by centrifuging at 3000 rpm for 10 min. The nitric oxide (NO) release levels and total antioxidant capacity (T-AOC) of HUVECs were tested.
Twenty male Bama minipigs (30–40 kg) fed a normal diet were used in this study in accordance with the guidelines of the Chinese Animal Care and Use Committee standards. Three days before surgery, 150 mg aspirin and 300 mg clopidogrel were administered orally with feed. Before surgery, an intramuscular injection of xylazine hydrochloride (0.15 mL/kg, 30 mg/1.5 mL) was given to each minipig for muscle relaxation. The venous channel was set up at the edge of the ear vein, and blood was taken simultaneously for a routine blood examination and serum biochemical examination, followed by ear intravenous administration of propofol at 8 ml/h for anesthesia. Minipigs were fixed to the operating table in a lateral position, and a 6F introducer sheath was positioned in the right femoral artery under surgical exposure. Heparin sodium (200 IU/kg) was injected via the sheath. All catheters were subsequently introduced through the sheath and advanced to the left anterior descending (LAD)/left circumflex (LCX)/right coronary artery (RCA) through a guide wire. Baseline angiograms of each artery were obtained for each pig through quantitative coronary analysis (QCA). Prepared stents were deployed in the suitable vessel region during angiographic guidance. It was ensured that no branches were present in the stented region. Stents were dilated at 10 atm. At the level of the balloon dilatation, the diameter of the aorta was approximately 2.5 mm and the expansion ratio of the aorta was 1:1.1. Two different stents were deployed per pig, and pre-deployment and post-deployment digital subtraction angiography was recorded. Gentamicin sulfate (5 mg/kg) was given as intramuscular injection in the next three days, and 150 mg aspirin and 300 mg clopidogrel were administered orally with feed every day. Animals were fed a normal diet after the intervention.
Restenosisrate(%)=1−lumenareainternalelasticlaminaarea×100%
Venous blood of pigs was collected for routine blood examination and serum biochemical examination.
Click Here to read full article.
Authors:
 Open Access
This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included
in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Open Access
This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included
in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Introduction
Cardiovascular
diseases are among the most common diseases that give rise to
disability and mortality. Currently, the use of coronary stents is
commonplace in the treatment of such diseases1.
Extensive data from experimental and clinical studies confirm that
although drug-eluting stents (DESs) reduce the rate of restenosis
compared with bare-metal stents (BMSs), there remains an increased risk
of late stent thrombosis with DESs2,3,4,5,6.
Percutaneous coronary intervention (PCI), especially involving stents,
usually results in injury to the endothelium, consequently inducing the
activation and gathering of blood platelets to form thrombosis, and the
stent may become a site for the adhesion of platelets before they are
completely covered by neointima4,7.
Other long-term clinical studies have reported that DESs loaded with
anti-proliferative agents suppressed the growth of neointima, delayed
the re-endothelialization of stents, lengthened the time for activation
of platelets, and increased the risk of late thrombosis8. Hence, the design of DESs in the future must consider these critical issues.
The anti-proliferative drugs rapamycin (also known as sirolimus) and paclitaxel were the first-generation drugs coated with DES approved by the US FDA. Subsequently, as newer-generation stents have emerged, sirolimus analogues such as zotarolimus and everolimus have been utilized. Despite these advancements, today’s DES have not completely eradicated restenosis and still lead to a higher incidence of late stent thrombosis. Drug docetaxel (DTX) is a paclitaxel analogue with enhanced anti-proliferative ability and water solubility and has been shown to be a good candidate drug for the prevention of restenosis9,10. Thrombosis remains a concern after stent implantation, whether at the early or very late stage. The exposure of damaged endothelial cells to the blood activates platelet adhesion and aggregation. The binding of the monoclonal antibody (mAb) to the platelet GP IIb/IIIa receptor11 has potent anti-platelet and anti-thrombotic characteristics that have been shown to reduce thrombosis-related major complications after coronary angioplasty12,13,14.
Polymer particles can be employed to deliver medication in a rate-controlled and sometimes targeted manner by drug leaching from the polymer or by degradation of the polymer matrix15. The advancement and convergence of particle drug delivery systems have recently been explored to optimize stent coatings. For example, drugs exhibiting different therapeutic effects were encapsulated in bi-layered PLGA particles containing vascular endothelial growth factor (VEGF) plasmid and paclitaxel; stents with this particle coating promoted early endothelium healing and inhibited smooth muscle cell (SMC) proliferation16,17,18. Local particle-based drug delivery was hypothesized to facilitate high regional concentrations of therapeutic agents with prolonged retention at low doses, leading to reduced systemic toxicity19,20. For drug loading and release of particles, there already exist several approaches21. Traditional particle preparation methods cannot adequately control the burst release of drugs22. Electrospraying, on the other hand, provides a superior alternative to producing stent coatings with different nano/micro particle sizes and assembly methods. Thian et al.23 used electrospraying deposition to produce a uniform coating consisting of hydroxyapatite nanocrystals on a titanium substrate, which triggered an early apatite precipitation process in cellular-simulated body fluid. Almería et al.24 demonstrated a multiplexed electrospraying process for the single-step synthesis of stabilized polymer particles for drug delivery, but the release rate of the drugs was found to be rapid, reaching approximately 70% within the first 4 h. To the best of our knowledge, only a limited number of studies have reported the application of electrospraying for the fabrication of vascular DES coatings. In particular, Dong et al. fabricated and controlled the release of the electrosprayed ReoPro-loaded metal vascular stent. Hydrophilic ReoPro release was controlled in the hydrophobic PLGA-based release system and was effective in suppressing platelet adhesion and SMC overgrowth for vascular stents25. Zamani’s research showed that multi-drug coatings could easily be fabricated using electrospraying. The electrosprayed Montelukast/poly(lactic-co-glycolic acid) particle-based coating is a potential new therapeutic approach toward the prevention of in-stent restenosis26.
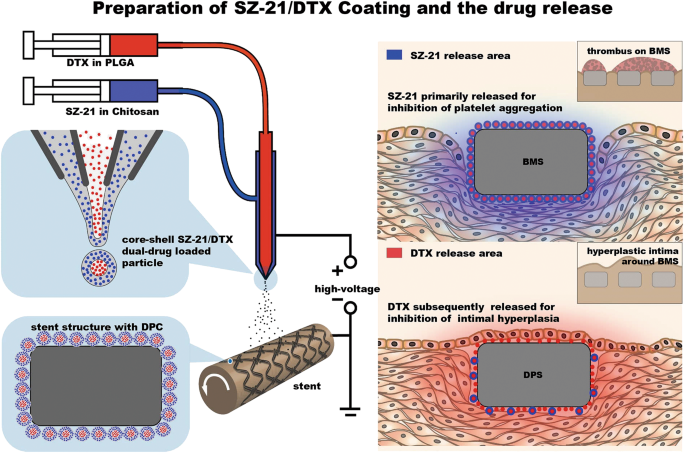
Ideal vascular implant coatings should exhibit a number of properties. These properties include biocompatibility, anti-thrombogenicity, the ability to interface with anti-proliferative drugs to reduce platelet activation level and time, restenosis prevention and a favorable surface topography for re-endothelialization27,28. To achieve dual-functionalized vascular stents with dual drugs, researchers have chosen to combine various coating methods and multiple-step methods for coating preparation29,30. To the best of our knowledge, there is no published work that considers multi-hydrophilic/hydrophobic drug coating preparation for coronary stents with a single step. In our previous research, we reported the detection of sequential drug release of polymer-controlled core/shell nanoparticles prepared by coaxial electrospraying31. Here, we designed a novel DES coating consisting of particles with a core of the anti-proliferative drug DTX and a shell of SZ-21, which was the platelet glycoprotein IIb/IIIa receptor monoclonal antibody32,33. The particles were produced and deposited on the stent by coaxial electrospraying (Fig. 1).
Fig. 1: Schematic diagrams of material design and function hypothesis.
The anti-proliferative drugs rapamycin (also known as sirolimus) and paclitaxel were the first-generation drugs coated with DES approved by the US FDA. Subsequently, as newer-generation stents have emerged, sirolimus analogues such as zotarolimus and everolimus have been utilized. Despite these advancements, today’s DES have not completely eradicated restenosis and still lead to a higher incidence of late stent thrombosis. Drug docetaxel (DTX) is a paclitaxel analogue with enhanced anti-proliferative ability and water solubility and has been shown to be a good candidate drug for the prevention of restenosis9,10. Thrombosis remains a concern after stent implantation, whether at the early or very late stage. The exposure of damaged endothelial cells to the blood activates platelet adhesion and aggregation. The binding of the monoclonal antibody (mAb) to the platelet GP IIb/IIIa receptor11 has potent anti-platelet and anti-thrombotic characteristics that have been shown to reduce thrombosis-related major complications after coronary angioplasty12,13,14.
Polymer particles can be employed to deliver medication in a rate-controlled and sometimes targeted manner by drug leaching from the polymer or by degradation of the polymer matrix15. The advancement and convergence of particle drug delivery systems have recently been explored to optimize stent coatings. For example, drugs exhibiting different therapeutic effects were encapsulated in bi-layered PLGA particles containing vascular endothelial growth factor (VEGF) plasmid and paclitaxel; stents with this particle coating promoted early endothelium healing and inhibited smooth muscle cell (SMC) proliferation16,17,18. Local particle-based drug delivery was hypothesized to facilitate high regional concentrations of therapeutic agents with prolonged retention at low doses, leading to reduced systemic toxicity19,20. For drug loading and release of particles, there already exist several approaches21. Traditional particle preparation methods cannot adequately control the burst release of drugs22. Electrospraying, on the other hand, provides a superior alternative to producing stent coatings with different nano/micro particle sizes and assembly methods. Thian et al.23 used electrospraying deposition to produce a uniform coating consisting of hydroxyapatite nanocrystals on a titanium substrate, which triggered an early apatite precipitation process in cellular-simulated body fluid. Almería et al.24 demonstrated a multiplexed electrospraying process for the single-step synthesis of stabilized polymer particles for drug delivery, but the release rate of the drugs was found to be rapid, reaching approximately 70% within the first 4 h. To the best of our knowledge, only a limited number of studies have reported the application of electrospraying for the fabrication of vascular DES coatings. In particular, Dong et al. fabricated and controlled the release of the electrosprayed ReoPro-loaded metal vascular stent. Hydrophilic ReoPro release was controlled in the hydrophobic PLGA-based release system and was effective in suppressing platelet adhesion and SMC overgrowth for vascular stents25. Zamani’s research showed that multi-drug coatings could easily be fabricated using electrospraying. The electrosprayed Montelukast/poly(lactic-co-glycolic acid) particle-based coating is a potential new therapeutic approach toward the prevention of in-stent restenosis26.
Ideal vascular implant coatings should exhibit a number of properties. These properties include biocompatibility, anti-thrombogenicity, the ability to interface with anti-proliferative drugs to reduce platelet activation level and time, restenosis prevention and a favorable surface topography for re-endothelialization27,28. To achieve dual-functionalized vascular stents with dual drugs, researchers have chosen to combine various coating methods and multiple-step methods for coating preparation29,30. To the best of our knowledge, there is no published work that considers multi-hydrophilic/hydrophobic drug coating preparation for coronary stents with a single step. In our previous research, we reported the detection of sequential drug release of polymer-controlled core/shell nanoparticles prepared by coaxial electrospraying31. Here, we designed a novel DES coating consisting of particles with a core of the anti-proliferative drug DTX and a shell of SZ-21, which was the platelet glycoprotein IIb/IIIa receptor monoclonal antibody32,33. The particles were produced and deposited on the stent by coaxial electrospraying (Fig. 1).
Materials and methods
Materials
Three hundred sixteen liters (316L) stainless steel (SS) stents (3.0 × 17 mm) and 316 L SS sheets (Ø 10 × 1.5 mm) were purchased from Beijing AmsinoMed Medical Device Co., Ltd., China. SZ-21 was supplied by Prof. Changgeng Ruan in the Jiangsu Institute of Hematology (First Affiliated Hospital of Suzhou University, Suzhou, China). Human umbilical vein endothelial cells (HUVECs) and Human umbilical arterial smooth muscle cells (HUASMCs) were a generous gift from Dr. Lushan Liu (Nan Hua University, China). DTX (Xieli Pharmaceutical Co., Ltd, Sichuan, China), chitosan (CS) Mw = 600,000 (Boxin Biotech Co., Ltd, Tianjin, China) and poly [(50% lactic acid) (50% glycolic acid)] (PLGA) Mw = 20,000 (Sigma Inc., St. Louis, USA) were also used in these studies.Multilayer coating with hydrophobic core/hydrophilic shell nano/micro particles by electrospraying
Coating experiments were carried out on 316 L SS stents (3.0 × 17 mm) and 316 L SS sheets (Ø 10 × 1.5 mm) utilizing coaxial electrospraying. The design and experimental setup of the coating procedure are shown in Fig. 1.Dichloromethane and ethylic acid solution were used as the solvents for PLGA and CS, respectively. In the electrospray coating process, 10 mL syringes containing DTX-PLGA solution (DTX concentration at 1 mg/mL) or SZ-21-CS solution (SZ-21 concentration at 2 mg/mL) were connected with a coaxial nozzle. The reception distance between the tip of the needle to the coating surface was maintained at 5–15 cm; the voltage and inner/outer flow rate were changed to obtain a uniform coating (Table S1). The SZ-21/DTX drug-loaded hydrophobic core/hydrophilic shell particle coating (DPC) and no drug core/shell particle coating (BPC) were prepared according to the following selected parameters: 0.2 mL/h (inner) and 0.6 mL/h (outer), a PLGA concentration of 10 mg/mL, a CS concentration of 2.5 mg/mL, a reception distance of 5 cm, and a voltage of 14 kV. The morphology, core/shell structure, and particle size distribution were detected through a fluorescence microscope (IX81, Olympus, Japan), scanning electron microscope (SEM; Vega-LMH, Tescan, Czech), confocal microscope (SP8, Leica, German) and transmission electron microscope (TEM, JEM-2100F, JEOL, Japan).
Morphometric analysis of the SZ-21/DTX drug-loaded hydrophobic core/hydrophilic shell particle coating stent (DPS)
Fluorescence characterization of DPS
DPS was prepared by coaxial electrospraying. To identify the hydrophobic core/hydrophilic shell particle nature of the coating, 1 mg/mL fluorescein isothiocyanate (FITC) was added to the shell solution and 1 mg/mL rhodamine B was added to the core solution, allowing for observation by fluorescence microscope (IX81, Olympus, Japan).SEM and EDS test of DPS
After coating, DPS was expanded three times for 30 s each time by balloon (2.75 mm × 18 mm MAVERJCK2, Boston scientific) at 10 atm in vitro; the changes in morphology and element contents of BMS, DPS and after expansion were all determined using a scanning electron microscope (SEM; Vega-LMH, Tescan, Czech) and energy dispersive spectrometer (EDS; INCA x-sight 7557, Oxford Instruments, UK), respectively.Atomic force microscopy and water contact angle analysis of DPS
The morphology changes of stents with different coatings were characterized using an atomic force microscope (AFM; SPI3800N, SEIKO, Japan). The hydrophilicity alteration of different coating surfaces, as determined by the distilled water contact angle, was measured with a contact angle tester (DSA30, Krüss, Germany) at room temperature. Three different sites were selected randomly for each group.Fourier transform infrared spectroscopy (FTIR) detection of drugs and polymers for DPS
FTIR measurement was performed to analyze the chemical structure of the coaxial sprayed coatings before and after coaxial electrospraying. Using the squash method, KBr (100–200 mg) and solid samples (1–2 mg) were ground into micron powder and pressed into sheets (Ø 13 × 1.0 mm) for analysis.Drug release analysis in vitro
Detection of drug release in vitro
For drug release analysis, DPSs were transferred in 1 mL phosphate-buffered saline (PBS) in a constant temperature shaking incubator at 60 rpm/min and 37 °C. The concentrations of released drugs were monitored at pre-determined time points, and the fresh PBS was replaced each time. Moreover, the stent morphology after 0, 7, and 28 days was characterized by SEM. The SZ-21 release rate in vitro was tested using mice immunoglobulin IgG Elisa kits. DTX was assayed by HPLC (Agilent 1100, USA) with a C18 250 mm × 4.6 mm column. The mobile phase was acetonitrile: water (65:35). The sample was eluted at 1 mL/min at room temperature, and the absorbance was measured at 230 nm.Mathematical modeling of drug release in vitro
The large number of core/shell particles on the coating made it impractical to mathematically model each individual particle. Instead, we adopted a continuum approach and approximated the core/shell coating layer as a biphasic material, providing two independent routes of transport for each drug. These two routes could result from surface preparation methods and may correspond to the drug phases being either fully embedded within the coating layer (slow release) or connected to the surface by pores or other defects (fast release)34.Each route of transport was associated with a different effective transport speed characterized by a rate constant ki, i = 1,2. Assuming that the drug was initially uniformly distributed within each respective route, the strut was impermeable to drug transport, the release medium acted as an infinite sink and the drugs were dilute, and then considering drug transport within each route as a diffusion-dominated process, we derived an expression for the mass of each drug within the coating as a function of time, M(t):
(1)
, and r is the initial fraction of the total drug loading (M0) within each route.
Using the knowledge of the total drug loading for each drug and Eq. (1), we calculated the cumulative fraction of each drug that had been released as a function of time.
Blood compatibility evaluation
Blood was obtained from healthy volunteers in accordance with the protocol approved by the Blood Donation Law of the People’s Republic of China. To isolate fresh human platelet-rich plasma (PRP), the blood was centrifuged at 1200 rpm for 10 min, and the supernatant was collected. All sheets were incubated in 0.5 mL of PRP for 30 min at 37 °C, rinsed with PBS, fixed with 2.5% glutaraldehyde for 12 h at 4 °C and dehydrated for SEM35. The expression of platelet granule membrane glycoprotein CD62P was detected to evaluate the anti-platelet activation of the coating. The deformability of adherent platelets (type I-V) on coatings was analyzed, and the morphological indices were calculated via the following formula:36
(2)
The proliferation and migration of HUVEC and HUASMC on coatings
Cell culture
HUVECs and HUASMCs were cultured in RPMI 1640 and DMEM/F12 media (HyClone, USA), respectively, supplemented with 10% fetal bovine serum (FBS; Cambrex Corp, USA) and 1% P/S (Gibco Industries Inc, USA) at 37 °C under 5% CO2.HUVECs seeding and cell viability assay
A rotation culture device after autoclave sterilization was used to seed cells on the stent surface. BMS, BPS, and DPS were transferred to the rotation culture tubes after undergoing ultraviolet irradiation sterilization. Then, HUVECs were seeded in the tubes at 5 × 104 cells/tube density with 10 rpm/min for 12 h at 37 °C. Finally, the stents were gently taken out in an aseptic condition and cultured in 12-well plates at 37 °C under 5% CO238.After a 5-day culture, the stents were immobilized with 2.5% glutaraldehyde and dehydrated for morphological observation by SEM. The proliferation rates of cells on the stent surfaces were investigated with Cell Titer 96® Aqueous One Solution Cell Proliferation Assay after 1, 3, and 5 days. The relative growth of rate (RGR) was calculated by this formula:
(3)
After co-culturing for 1, 3, and 5 days, the culture supernatant was collected by centrifuging at 3000 rpm for 10 min. The nitric oxide (NO) release levels and total antioxidant capacity (T-AOC) of HUVECs were tested.
The migration of HUVECs and HUASMCs on coatings
HUVECs and HUASMCs were cultured separately. Both HUVEC and HUASMC migration assays were carried out with the classical scarification method. Different coatings were sprayed on 316 L SS sheets by coaxial electrospraying. Then, the sheets of three groups (316 L SS, BPC, DPC) were placed in 24-well plates after undergoing ultraviolet irradiation sterilization for 12 h. One milliliter of cell suspension (1 × 105 cells/ml) was seeded and grown on the coating surfaces. After being starved in media with 1% serum for 4 h, confluent monolayers of HUVECs were wounded by scraping a pipet tip (200 μL) across the monolayer to produce initial wounds with a constant diameter; they were then washed three times with PBS. The migration distance of the scratch was measured at 0, 6, 12, and 24 h using ImageJ (NIH, US). The migration lengths were counted following the methods in our previous research38.Stent implantation in vivo
Two groups of drug-coated stents were prepared using coaxial electrospraying: DPSs containing 50 ± 2.36 μg DTX (DPS-L) and DPSs containing 100 ± 3.88 μg DTX (DPS-H). The DTX content on the two stent groups was detected in the drug release after complete elution. Three control groups, BMS, BPS, and SES (DES with Sirolimus), were also prepared.Twenty male Bama minipigs (30–40 kg) fed a normal diet were used in this study in accordance with the guidelines of the Chinese Animal Care and Use Committee standards. Three days before surgery, 150 mg aspirin and 300 mg clopidogrel were administered orally with feed. Before surgery, an intramuscular injection of xylazine hydrochloride (0.15 mL/kg, 30 mg/1.5 mL) was given to each minipig for muscle relaxation. The venous channel was set up at the edge of the ear vein, and blood was taken simultaneously for a routine blood examination and serum biochemical examination, followed by ear intravenous administration of propofol at 8 ml/h for anesthesia. Minipigs were fixed to the operating table in a lateral position, and a 6F introducer sheath was positioned in the right femoral artery under surgical exposure. Heparin sodium (200 IU/kg) was injected via the sheath. All catheters were subsequently introduced through the sheath and advanced to the left anterior descending (LAD)/left circumflex (LCX)/right coronary artery (RCA) through a guide wire. Baseline angiograms of each artery were obtained for each pig through quantitative coronary analysis (QCA). Prepared stents were deployed in the suitable vessel region during angiographic guidance. It was ensured that no branches were present in the stented region. Stents were dilated at 10 atm. At the level of the balloon dilatation, the diameter of the aorta was approximately 2.5 mm and the expansion ratio of the aorta was 1:1.1. Two different stents were deployed per pig, and pre-deployment and post-deployment digital subtraction angiography was recorded. Gentamicin sulfate (5 mg/kg) was given as intramuscular injection in the next three days, and 150 mg aspirin and 300 mg clopidogrel were administered orally with feed every day. Animals were fed a normal diet after the intervention.
Acquisition and observation of stent implantation samples
Pigs were euthanized at 1, 3, and 6 months (n = 3 at each time point for each stent group). At each time point, aortic specimens were excised transversely into two parts. One part was fixed in 4% paraformaldehyde and embedded in light-cured resin with hematoxylin and eosin stain (HE) for pathology organization analysis. Another part was fixed in 0.25% glutaraldehyde for 48 h, and the morphology of the intima surface was observed by SEM. The proximal part and distal part of each stented vessel, myocardium, liver, spleen, lung, and kidney of pigs were fixed and sliced to check the safety of stents. Histomorphometric analysis was conducted on each section, including the vessel area (mm2), internal elastic lamina (IEL) area (mm2), lumen area (mm2), neointima area (mm2), and the neointima thickness (mm) by Image Tool. The percentage of restenosis was calculated using the following equation:
(4)
Statistical analysis
At least three independent experiments were performed for the tests described above. Statistical analyses were conducted with GraphPad Prism 6. The differences between experimental groups were considered statistically significant when p < 0.05. Unless indicated, the values are expressed as the mean ± SD.Click Here to read full article.
Authors:
0 Comments