Mechanical interactions between bacterial cells and extracellular polymeric substance are essential in determining biofilm assembly and disassembly as well the mechanical characteristics of biofilms. However, the physics of these mechanical interactions in different cell culture conditions are poorly understood. We created typical artificial biofilm consisting of planktonic bacteria and hydrogel, in the absence of metabolic or regulatory effect. We have demonstrated that the cell culture medium can significantly affect the mechanical interactions between bacterial cells and hydrogels. The stiffness of the bacteria-hydrogel artificial biofilm cannot be simply attributed by the summation of the contribution from the bacteria and hydrogel based on the mathematical models and computational models. We have revealed that the tryptone component of Luria-Bertani broth medium plays an important role in stiffening effect of bacteria-hydrogel construct. Such significant stiffening effect can be explained by the following mechanism: the presence of tryptone in cell culture medium may enable the bacteria itself to crosslink the hydrogel polymer chains. Our findings have also demonstrated the synergy of modelling and innovative experiments which would potentially impact the biofilm control strategies.
Introduction
All
living things interact with their external environment and are
susceptible to changes when the environment changes. Many studies have
been conducted on animal cells to establish how they are affected by
changes in the extracellular matrix (i.e. the environment), either in terms of two-dimensional interactions with surfaces1,2,3 or when cells are surrounded by an external 3D environment4,5,6,7,8,9.
The impact of these environments on cell metabolism, protein synthesis,
cytoskeletal architecture, cell motility, gene expression and cell
mechanical properties have been studied10,11,12,13.
Bacterial adhesion on surfaces has been widely investigated in many
contexts including biomaterial associated infections, for example, since
these interactions are considered to be the starting point for
colonisation and biofilm formation14,15,16,17,18. Interactions of bacteria with many different surfaces have been studied including hard surfaces such as metals18,19, and different types of hydrogels20,21
that are natural, synthetic or a combination of both. Due to their
aqueous environment, controllable mechanical and chemical properties and
porous structure, hydrogels are suitable environments to interact with
bacterial cells22,23. The chemical24,25 and physical properties24,26,27,28,29 of hydrogels, and environmental factors24,30
have been shown to affect bacterial growth rate and their ability to
adhere. However, there is a lack of studies on how bacterial cells may
be affected by a 3D micromechanical environment31
such as when bacteria penetrate soft tissues to form localised or
systemic infections. There are several studies on embedding bacterial
cells for 3D printing applications combining multiple types of bacterial
cells to study cell-cell interactions32 and a controlled spatial distribution and concentration33
but the information on the mechanical interactions between the
bacterial cells and hydrogels is limited. Therefore, this study aimed to
investigate how physical (i.e. stiffness) and chemical (i.e.
chemical composition of the growth environment) factors affect the
mechanical interactions between bacterial cells and hydrogels, and
bacteria cell mechanics when they are encapsulated in a 3D
micro-environment. Due to the increased use of hydrogels in biomedical
applications such as in implants (i.e. hydrogel coated cochlear implants)34 or indwelling medical devices (i.e. hydrogel coated venous catheters)35,
understanding these properties might help us to design new materials or
to select the most appropriate materials based on their interactions
with bacterial cells. In addition, bacteria encapsulated in hydrogels
have been used as artificial biofilm models36,37,38,
which could simulate some important physicochemical characteristics of
real biofilms and enable more reproducible results than the real
biofilms. Therefore, it is essential to study the mechanical
interactions between bacteria and hydrogels.
Results and Discussion
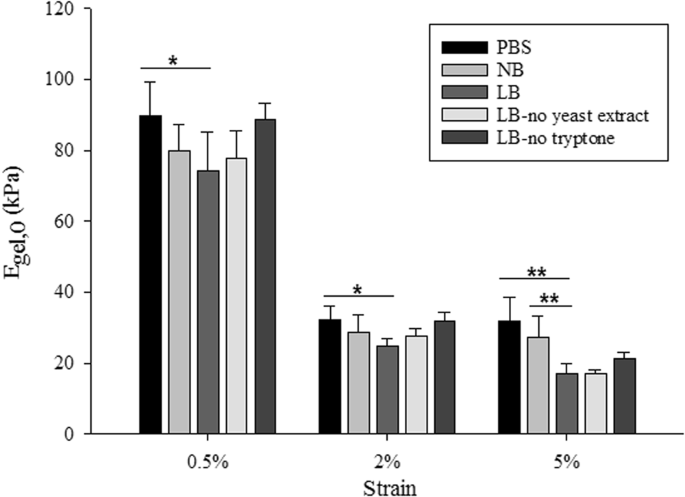
Stiffness of 1% agarose hydrogels without encapsulation at different strains
Agarose hydrogels were chosen as the matrix material due to their biocompatibility, inertness, gelling behaviour and the large content of water which favours bacterial cell hydration. In addition, previous work has demonstrated that agarose as a matrix polysaccharide was appropriate to simulate the extracellular polymeric matrix39. The stiffness of 1% agarose gels made with phosphate buffered saline (PBS), bacterial culture media (Luria-Bertani Broth (LB)) or distilled water has been reported in several studies23,40,41,42. These values were obtained from different mechanical tests, including simple tensile or compression tests, rheological characterisation or atomic force microscopy (AFM), and the range of the reported values varied between 14 kPa – 60 kPa. In addition to different constituents used and the various testing techniques, mechanical properties of agarose hydrogels also depended on the preparation protocols42.Measuring the stiffness of hydrogels infused with different growth media or buffer without bacterial encapsulation provided the basis of the measurements as the stiffness values measured were compared to the stiffness of gels with encapsulation (below). The instantaneous elastic moduli of 1% agarose hydrogels without encapsulation were obtained (Fig. 1) based on Hooke’s law (i.e. instantaneous modulus = maximum stress (σ) divided by the strain (ε)). When the loading time is much shorter compared to the relaxation characteristic time constant, Hooke’s law is a reasonable approximation at the given strains in this study. For all tested strains, 1% agarose gels made with PBS were stiffer than the gels made with growth media, suggesting that the medium used to form the hydrogel affects the stiffness of the gel. When the stiffness of the gels made with buffer and culture media (i.e. LB and NB) were compared, PBS gels were stiffest, followed by gels made with NB and then those made with LB. For all applied strains, PBS gels were significantly stiffer than LB gels (p < 0.05 for 0.5% and 2% applied strain and p < 0.01 for 5% applied strain) but there were no significant differences observed between PBS and NB gels. At lower strains (0.5% and 2%), there were no statistically significant differences between the stiffness of gels made with LB or NB. By contrast, at 5% strain, gels containing NB were significantly stiffer than those containing LB (p < 0.01).
A strain dependent behaviour of stiffness was observed for the tested hydrogels. For instance, the apparent stiffness of LB gels decreased with the increasing applied strain, i.e. the stiffness of the gel was obtained as 74.2 kPa, 24.8 kPa and 17.1 kPa when the applied strain was increased from 0.5% to 2% and 5% respectively. As reported previously43,44,45, several polymers have shown strain softening effect similar to the classical model of rubber elasticity, where the apparent stiffness of the material decreases with the increasing applied strain.
For PBS, NB and LB gels without encapsulation, there was a significant decrease (p < 0.01) in stiffness values when the applied strain was changed from 0.5% to 2%, which could be due to the collapse of the porous structure of the hydrogels, similar to what has been reported in many other porous materials46,47,48. However there were no significant differences (p > 0.05) in the measured stiffness when strain changes from 2% and 5%, which suggests that the stress-strain curve of these hydrogels reached a plateau in this strain region.
It was evident that the presence of tryptone weakened the hydrogel as measured at different applied strains (see Fig. 1). Interestingly, the stiffness of hydrogels made with LB, LB-no tryptone or LB-no yeast extract decreased by 76–78% when the applied strain increased from 0.5 to 5%. For PBS and NB gels, the stiffness decreased only by 66% when the applied strain increased from 0.5 to 5%.
Stiffness of hydrogels with bacteria
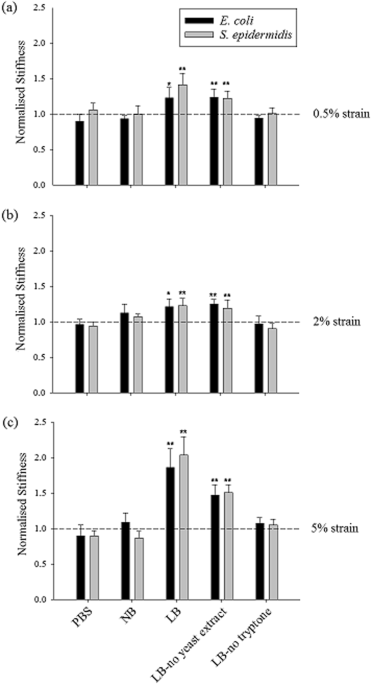
Having measured the stiffness of hydrogels alone, the next step was to assess whether the encapsulation of bacteria in the gels led to any measurable changes in the stiffness. Therefore, E. coli or S. epidermidis were encapsulated at a concentration equivalent to 1% of the total hydrogel volume. A similar stiffness characterisation was carried out for gels with encapsulated cells and the obtained stiffness values were normalised by the corresponding gel without encapsulation. The stiffness values of gels with encapsulated bacterial cells are given in Table S1. 1% LB gels with encapsulated E. coli and S. epidermidis cells were stiffer than 1% LB gels without bacteria. Interestingly, for the 1% gels made with PBS and NB, such an increase in stiffness was not observed and they showed similar stiffness values as the gels without bacteria (Fig. 2a–c). The significantly higher stiffness of LB gels with encapsulated bacteria compared with LB gels without bacteria could be attributed to the interactions between the bacterial cells and the media used to prepare the hydrogel. To investigate this further, experiments were performed to determine which constituent of LB medium, tryptone or yeast extract, was causing this increase in stiffness. Different media were prepared in which either constituent was omitted, for LB-no yeast extract gels yeast extract was omitted and for LB-no tryptone gels tryptone was omitted. The salt content was not changed since NaCl was present also in NB and PBS, and therefore could not be solely responsible for the observed differences in hydrogel stiffness between agarose formulations. The stiffness of these gels with and without bacteria was calculated and normalised. Only LB-no yeast extract gels with bacteria showed significant increases in normalised stiffness when bacterial cells were encapsulated, which possibly suggests that bacterial surface properties may have been altered in response to the peptides present in tryptone (Fig. 2a–c). Both types of bacterial cells (E. coli – rod shaped and S. epidermidis – spherical shaped) behaved in a similar pattern suggesting that different bacteria interact similarly with the media and the applied mechanical stimuli.The force generated by bacterial cells might cause a change in pore size in agarose gel, resulting in differences in the overall stiffness of the bacteria/hydrogel composite. However, such a mechanism in altering pore size of agarose gel is unlikely to be wholly responsible for the dramatic stiffening effect of some bacteria/hydrogel composites as seen in Fig. 2, provided the fact that the volume fraction of bacterial cells is so low (up to 1%). A significant increase in hydrogel stiffness is usually caused by chemically induced cross-linking of polymer chains that form the hydrogel network51. Particle reinforced hydrogels have also been reported elsewhere. For example, it was observed that bioactive glass particles can increase the stiffness of the polysaccharide gellan gum hydrogel by ~ 100 times at 2% concentration of particles52. Such a huge stiffening effect cannot be explained by the composite theory but could indicate crosslinking of the loose polymer chains by the particles. In this study, a similar mechanism may apply where bacterial cells crosslink the loose polymer chains. Also, it has also been demonstrated that the stiffness of material can affect the biological activities of bacterial cells when these cells are seeded on the material surface27,53 and such an effect can take place when bacterial cells are encapsulated inside hydrogels as well.
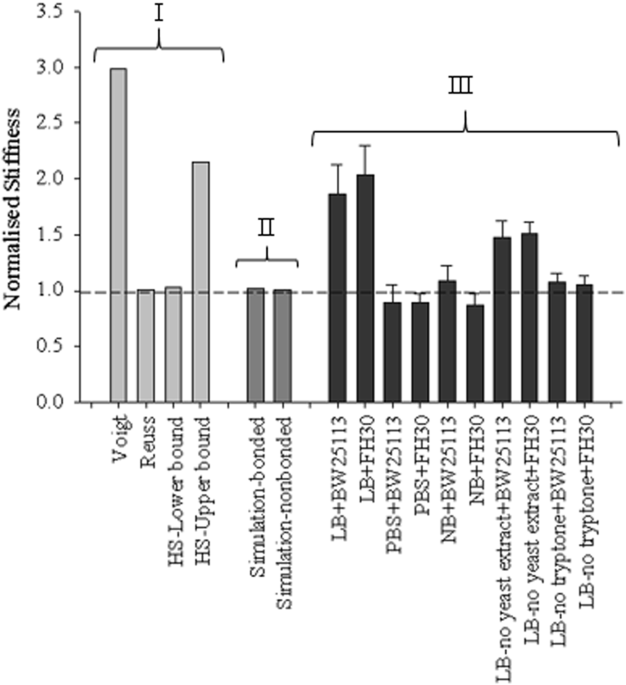
Computational modelling in comparison to mathematical models
Composite materials are made by combining two or more materials having different properties in a way that they do not dissolve or blend in each other to obtain a new material with unique properties54. In this sense, hydrogels with encapsulated cells or particles may be treated as composites providing the mentioned composite characteristics. In the literature, there are several mathematical models, including the Voigt model, Reuss model, and Hashin and Strikman model with upper and lower bounds (i.e. limits), to determine composite stiffness54,55,56. These models were employed to establish whether the experimental data and the simulation data would match at the tested volume of 1%.A computational model was developed to represent the compression tests for the case where the encapsulated particles do not interact with their environment (i.e. inert particles). Several variables were considered in this model, namely the applied compressive strain, the volume fraction of particles, Young’s modulus and Poisson’s ratio of the particle and the matrix, so that the model correctly represented the compression test conditions. It was reported in a previous study57 that the whole and live E. coli cells have a Young’s modulus of 2-3 MPa. This stiffness value was approximately 200 times as the hydrogel Young’s modulus41. The individual matrix and particle stiffness values were chosen so that they represented the same fold difference between the particle (bacteria) and the matrix (hydrogel). The same fold difference was also employed for the mathematical models. Providing a similar applied strain value and volume fraction of particles (1%) allowed comparisons with the experimental results. Young’s modulus was calculated based on the force value applied to the composite when the applied strain value reached 5%.
An incompressible material has a Poisson’s ratio of 0.558. When hydrogels are fully swollen their properties resemble rubber like materials59 which are highly incompressible and have a Poisson’s ratio close to 0.5. Similarly, bacterial cells are rigid structures featuring viscoelastic properties23 and their adapted Poisson’s ratio were documented between 0.4–0.5 in several studies60,61,62,63. Therefore, in the Hashin and Strikman models and in the computational model, both the matrix and the particle Poisson’s ratios were selected as 0.45.
The mathematical model values, simulation values and the experimental data obtained from compression tests were plotted together for comparison (Fig. 3). The results obtained from both bonded and non-bonded simulation were almost completely coincident with the Reuss model/HS-lower bound. There were two different groups of experimental data at 1% volume fraction: the first group was located between Voigt model/HS-upper bound and Reuss model which consisted of data points from LB and LB-no yeast extract gels (where significant differences were observed between the gels with and without encapsulation) and the second group was closer to Reuss model/HS-lower bound which consisted of data points belonging to 1% gels made with PBS, NB and LB-no tryptone (where no significant differences were observed between the gels with and without encapsulation).
Bacteria growth in 1% agarose gels made with different growth media
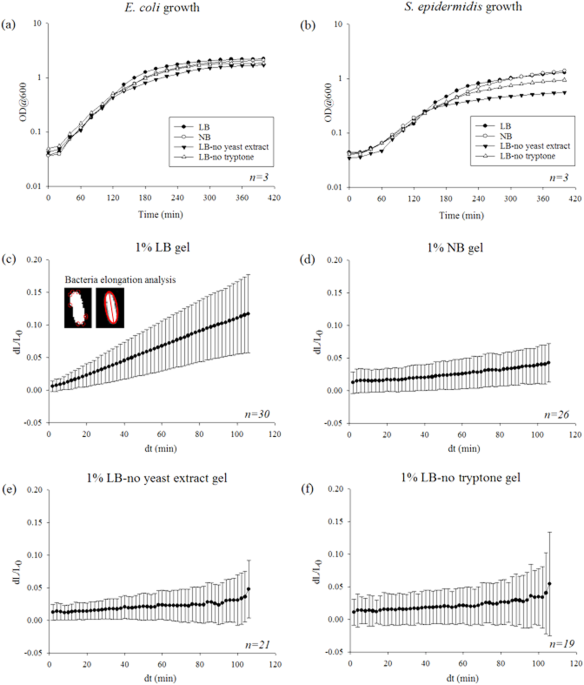
Batch growth curves were obtained for E. coli and S. epidermidis in the growth media used to make the hydrogels (LB, NB, LB-no yeast extract and LB-no tryptone, respectively) to obtain their growth behaviour in an environment where no hydrogel forces were acting on them (Fig. 4a,b). During exponential phase there were no significant differences between growth rates when different growth media were used for both E. coli and S. epidermidis cells. However, the lag phase of S. epidermidis cells was longer compared to that of E. coli cells for all the growth media considered. Bacteria growth in liquid media did not affect the overall growth behaviour of the bacterial cells when the growth took place without any forces acting on them by the hydrogels (i.e. when they were not encapsulated). The doubling time for E. coli cells was measured as 20 min and the doubling time for S. epidermidis cells was measured as 40 min during exponential phase.In addition, the doubling time of the encapsulated cells were calculated by fitting a curve to the data. From the fit, doubling time of E. coli cells in LB gels, NB gels, LB-no yeast extract gels and LB-no tryptone gels were calculated as 15.2 h, 60.5 h, 80.3 h and 69.4 h respectively. The reason for the long doubling time may be due to the bacterial cells not being able to reach exponential phase or grow at all due to the reaction forces acting on them from their 3D micro-environment, especially when certain growth media were used. It may also be limited by nutrient diffusion, which presumably will be much slower in a hydrogel than in broth. As the bacterial cells have such slow growth rates in hydrogels made with different media, it is unlikely that volume changes caused by growth in media could have led to significant increases in the stiffness of the bacteria/gel composites with the timeframe of the mechanical tests. Our computational simulations also confirmed this since the modelled encapsulated particles did not change their volume in time and the difference between the gel with particles and without particles did not show any significant differences for both bonded and non-bonded cases (see Fig. 3). Therefore, the key mechanisms to answer for this stiffening effect is likely due to cell-materials interactions when cells are in contact with the hydrogels with different physical/chemical properties. It is known that bacterial cells change surface characteristics to adapt to nutrient availability64 which would affect how they interact with their environments. In addition, different sources of tryptone can affect the physiological state of bacterial cells even in liquid medium65. Similar effects can apply to bacterial cells encapsulated inside hydrogels. In addition, it has been shown that different cell-materials interactions affected the cell membrane, cell phenotype and other physiological conditions of bacteria when bacterial cells are in contact with two-dimensional materials surfaces66. All these may also happen when bacterial cells are in contact with three-dimensional materials.
Conclusions
In
summary, this study employed an array of computational and experimental
approaches to explore how the three-dimensional biomaterials can affect
bacteria. It is found that the presence of tryptone tends to weaken
agarose hydrogels. However, the presence of tryptone in culture medium
apparently enhances the stiffness of the hydrogel when bacterial cells
are present, possibly by enabling bacteria to crosslink the polymer
chains in agarose. This may imply that tryptone is capable to trigger
some unknown effect on bacterial surface characteristics. However,
tryptone alone could not support cell growth unless yeast extract was
also present in LB medium. The majority of previous work has only
reported that the nutrient and materials stiffness would affect
bacterial growth on materials surfaces. Our study revealed that
biophysical parameters of materials can also regulate biophysical
responses of bacteria that are encapsulated inside hydrogels. Such
mechanisms may also be extended to biofilms because the hydrogel matrix
mimics the extracellular polymer produced in a biofilm. Thus, changes in
extracellular polymer matrix caused by factors such as chemicals or
mechanical stresses may regulate bacteria mechanics, which potentially
may lead to changes in cell structure and metabolic functions. Future
studies will aim to investigate the impact of different 3D
microenvironments on transcription and protein expression in bacteria,
to better understand the potential for the control of biofilms by
modulation of the extracellular matrix.
Authors:
 Open Access
This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included
in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Open Access
This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included
in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Authors:
0 Comments